When you pick up a pill, you’re probably thinking about the drug inside it-what it does, how fast it works, whether it’ll help your headache or high blood pressure. But what you can’t see? The rest of the pill. More than 90% of that tablet or capsule isn’t the active ingredient at all. It’s made up of things like starch, sugar, dyes, and preservatives. And while these are called "inactive," that label is starting to look more and more misleading.
What exactly is an active ingredient?
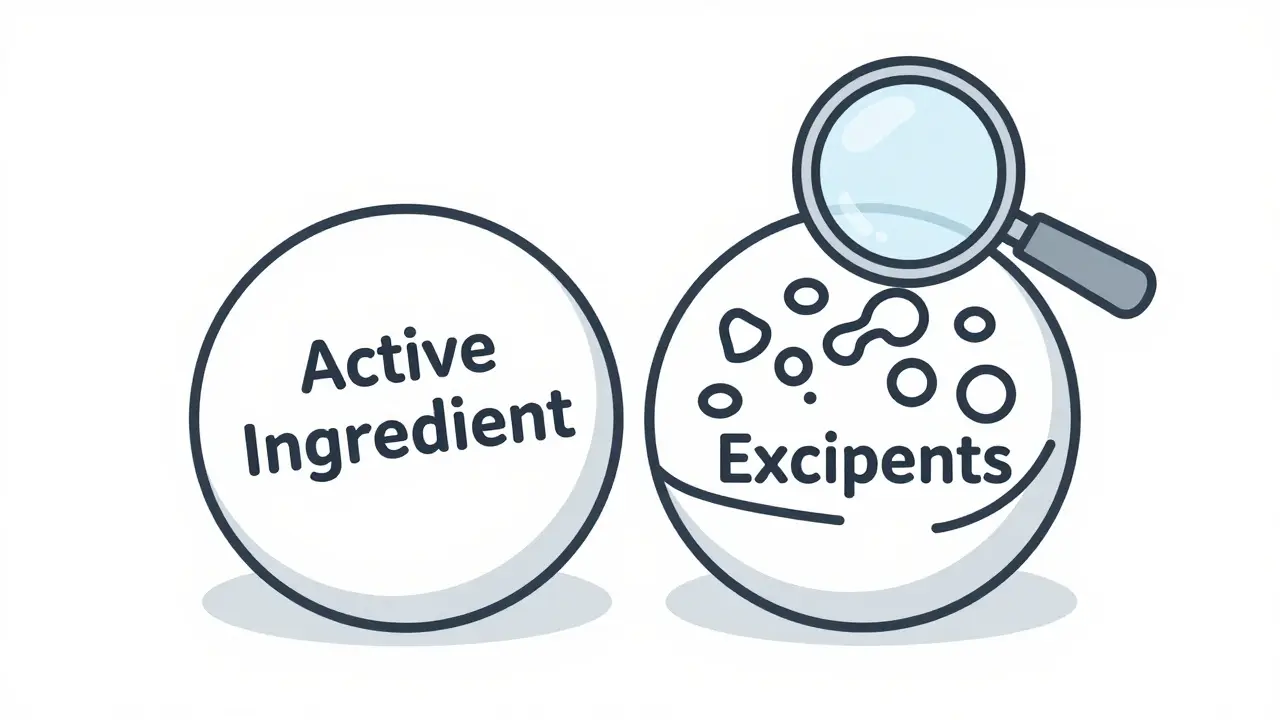
The active ingredient is the part of your medicine that actually does the job. It’s the molecule that talks to your body-binding to receptors, blocking enzymes, or changing how cells behave. Take Tylenol: its active ingredient is acetaminophen. Advil? That’s ibuprofen. Lipitor? Atorvastatin. These aren’t just names on the label-they’re precise chemicals with known effects, tested in years of clinical trials to prove they work and are safe at prescribed doses.
The U.S. Food and Drug Administration (FDA) defines active ingredients as components that "intend to furnish pharmacological activity or other direct effect" on your body. That means if you take a pill for high cholesterol, the active ingredient is the one lowering your LDL. If you’re taking an antibiotic, it’s the one killing bacteria. Without this part, the pill is just a fancy piece of chalk.
So what are inactive ingredients-and why do they exist?
Inactive ingredients, also called excipients, are everything else in the medicine. They don’t treat your condition. But they’re absolutely necessary to make the medicine work properly. Think of them as the support crew behind the star player.
- Fillers like lactose or microcrystalline cellulose give bulk to tiny doses. If a drug needs only 5 milligrams to work, you can’t just swallow a grain of powder. Fillers make the pill the right size.
- Binders like gelatin or acacia hold everything together so the tablet doesn’t crumble.
- Lubricants such as magnesium stearate prevent the medicine from sticking to factory machines during mass production.
- Coatings like hydroxypropyl methylcellulose help you swallow the pill and control how fast the drug releases in your gut.
- Preservatives like parabens stop bacteria or mold from growing in liquid medicines or multi-dose vials.
- Flavors and colors make it easier for kids or adults to take their medicine regularly. A cherry-flavored syrup is more likely to be taken than a bitter, gray liquid.
The Australian Department of Health says inactive ingredients do four key things: fill out small doses, keep the active ingredient stable, help your body absorb it better, and make the medicine easier to take. Without them, most medications wouldn’t be practical-or even safe-to use.
But "inactive" doesn’t mean harmless
Here’s where things get surprising. A 2021 study from the National Institutes of Health (NIH), led by researchers at UC San Francisco and Novartis, tested 639 commonly used inactive ingredients against over 3,000 human proteins. They found that 14% of these "inactive" substances showed biological activity-meaning they interacted with your body’s systems in ways no one expected.
Some compounds, like D&C Red 7 calcium lake (a red dye) and propyl gallate (a preservative), bound strongly to proteins involved in inflammation, metabolism, and even hormone regulation. These weren’t random flukes. The researchers tested them at concentrations you’d actually find in a pill-and the effects were measurable.
This challenges the old idea that excipients are "inert." They might not cure your pain, but they could still be influencing your body. For most people, this doesn’t matter. But for someone with a chronic condition, a sensitive immune system, or a genetic variation in how they process chemicals, these hidden interactions could add up.
Why this matters for real people
Let’s say you have lactose intolerance. About 65% of people worldwide have trouble digesting lactose. If your generic blood pressure pill uses lactose as a filler, you might get bloating, gas, or diarrhea-even though the active ingredient is perfectly safe. You might think it’s your diet, but it’s the pill.
Or consider celiac disease. About 15% of people with gluten sensitivity unknowingly take pills with wheat starch as a binder. That’s enough to trigger symptoms-even if the drug itself is fine.
And it’s not just allergies. Some inactive ingredients can change how well your body absorbs the active drug. Take fenofibrate, a cholesterol-lowering drug. A newer version with specific surfactants increased absorption by 35% compared to older formulations. That’s not a small difference-it can mean the difference between your treatment working or failing.
Even more alarming? The FDA’s own adverse event data shows that 0.5% of all reported drug reactions are linked to inactive ingredients. That might sound low, but with billions of pills taken every year, that’s thousands of people experiencing side effects because of something they never knew was in their medicine.
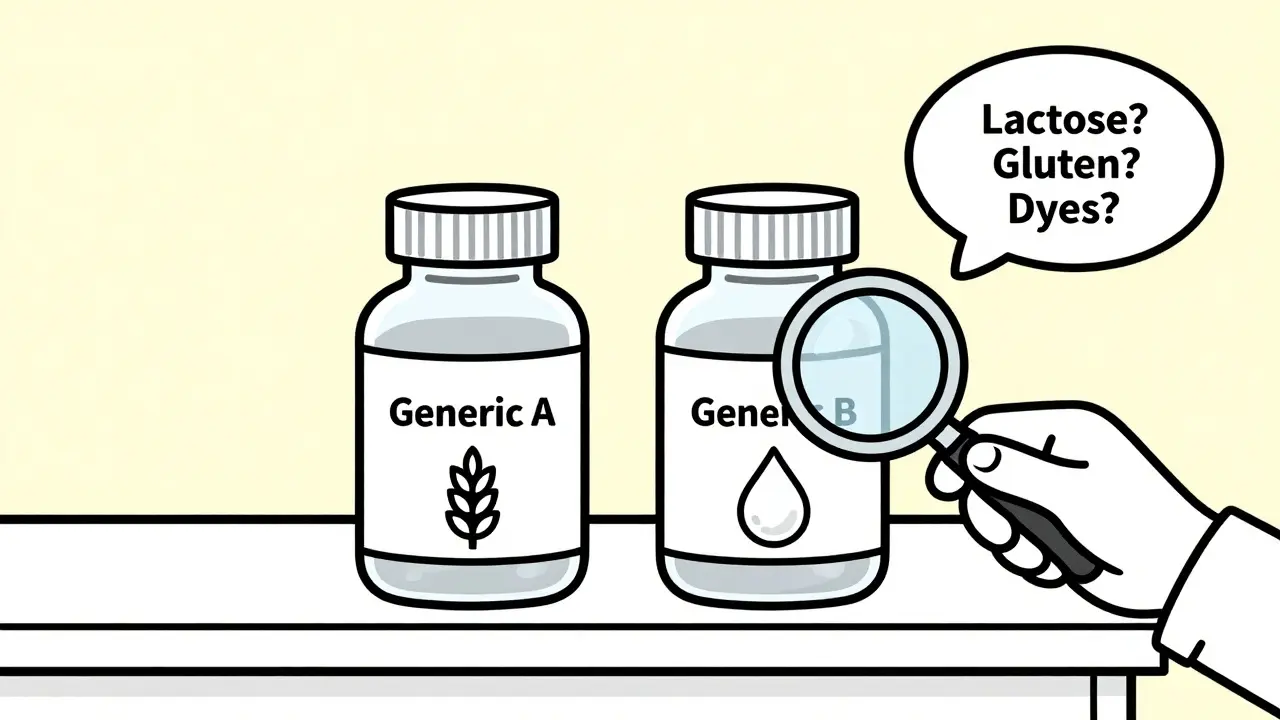
What’s changing in the industry
The pharmaceutical world is starting to wake up. The FDA launched its Excipient Safety Initiative in 2022, pouring $4.2 million into research to better understand these hidden effects. Sixty-eight of the top 100 drug companies now use computational tools-similar to the NIH study-to screen excipients for biological activity before they’re used in new drugs.
Regulators are updating rules, too. The FDA now requires more testing for excipients used in high-dose or long-term medications. And in Australia, since 2020, pharmacists must prescribe by active ingredient-not brand name. That means you get the same drug, but you can ask for a version without lactose, gluten, or artificial dyes.
Meanwhile, the global market for excipients is booming. It was worth $7.8 billion in 2022 and is expected to hit $11.3 billion by 2027. Why? Because smart drug design isn’t just about the active ingredient anymore. It’s about the whole package.
What you can do
You don’t need to be a chemist to protect yourself. Here’s how to take control:
- Check the label. All OTC drugs list inactive ingredients on the back. Prescription meds include them in the package insert. Look for things like "lactose," "corn starch," "FD&C Red #40," or "sulfites."
- Ask your pharmacist. If you have allergies, sensitivities, or digestive issues, ask if there’s a version of your medicine without the problematic ingredient. Many drugs come in multiple formulations.
- Use the FDA’s Inactive Ingredient Database. It’s public, free, and updated quarterly. You can search by ingredient or drug form to see what’s approved and at what levels.
- Don’t assume generics are identical. Same active ingredient? Yes. Same fillers? Not always. One generic might use gluten-containing starch; another might use rice flour. The difference matters.
Medicine isn’t just about the molecule that treats your disease. It’s about the whole system that delivers it. Ignoring the "inactive" ingredients is like only checking the engine of your car and ignoring the fuel, oil, and spark plugs. They might not "drive" you-but they make sure you don’t break down on the way.



Hariom Sharma
I never thought about this before, but yeah, my stomach has been acting up since I switched to a generic blood pressure med. Turns out it had lactose in it. My pharmacist was shocked I didn’t know. Now I always check the label-life-changing stuff. India’s got tons of people with lactose issues too, and no one talks about it. We need more awareness!