Enzalutamide Survival Calculator
How This Calculator Works
Based on clinical trial data, this tool estimates the potential overall survival benefit of Enzalutamide for prostate cancer patients. Input patient characteristics to see expected median survival difference compared to placebo.
Patient Characteristics
Estimated Survival Benefit
Enter patient characteristics to see estimated benefit
Note: This calculator provides estimates based on clinical trial data. Individual results may vary. Always consult with a healthcare provider for treatment decisions.
Quick Takeaways
- Enzalutamide is an androgen receptor inhibitor that prolongs overall survival in metastatic castration‑resistant prostate cancer (mCRPC).
- Key trials - AFFIRM (post‑chemotherapy) and PREVAIL (chemotherapy‑naïve) - showed 3‑ to 4‑month median OS gains.
- Patients with low AR‑V7 expression and good performance status benefit most.
- Common side effects include fatigue, hypertension, and seizures (rare).
- Standard dose is 160 mg daily, taken with continuous androgen‑deprivation therapy.
When you hear about a new drug for prostate cancer, the first question is usually: *Does it actually help people live longer?* The answer for Enzalutamide is a clear yes-if the right patients are chosen and the treatment is managed well. Below we unpack how the drug works, what the biggest trials have shown, who should consider it, and what to watch out for along the way.
What is Enzalutamide?
Enzalutamide is a third‑generation androgen receptor (AR) inhibitor approved for treating various stages of prostate cancer, especially metastatic castration‑resistant prostate cancer (mCRPC). By binding to the AR, it blocks testosterone‑driven signaling, prevents the receptor from moving into the cell nucleus, and stops cancer cells from growing. The drug was first approved by the FDA in 2012 and has since become a cornerstone of hormone‑based therapy.
How Does Enzalutamide Work?
Prostate cancer cells rely on the AR to survive. Traditional androgen‑deprivation therapy (ADT) lowers testosterone levels, but cancer can adapt by over‑expressing the receptor or mutating it. Enzalutamide tackles this adaptation in three steps:
- It binds to the ligand‑binding domain of the AR, blocking testosterone and dihydrotestosterone from attaching.
- It prevents the AR from translocating into the nucleus, where it would normally turn on growth‑promoting genes.
- It stops the AR from recruiting co‑activators, shutting down the transcription of cancer‑driving genes.
Think of it as a triple‑lock on a door that cancer has learned to pick. By locking the AR in three places, the drug makes it far harder for the tumor to keep growing.
Clinical Evidence: AFFIRM and PREVAIL Trials
Two pivotal Phase III trials give us the hard data on survival:
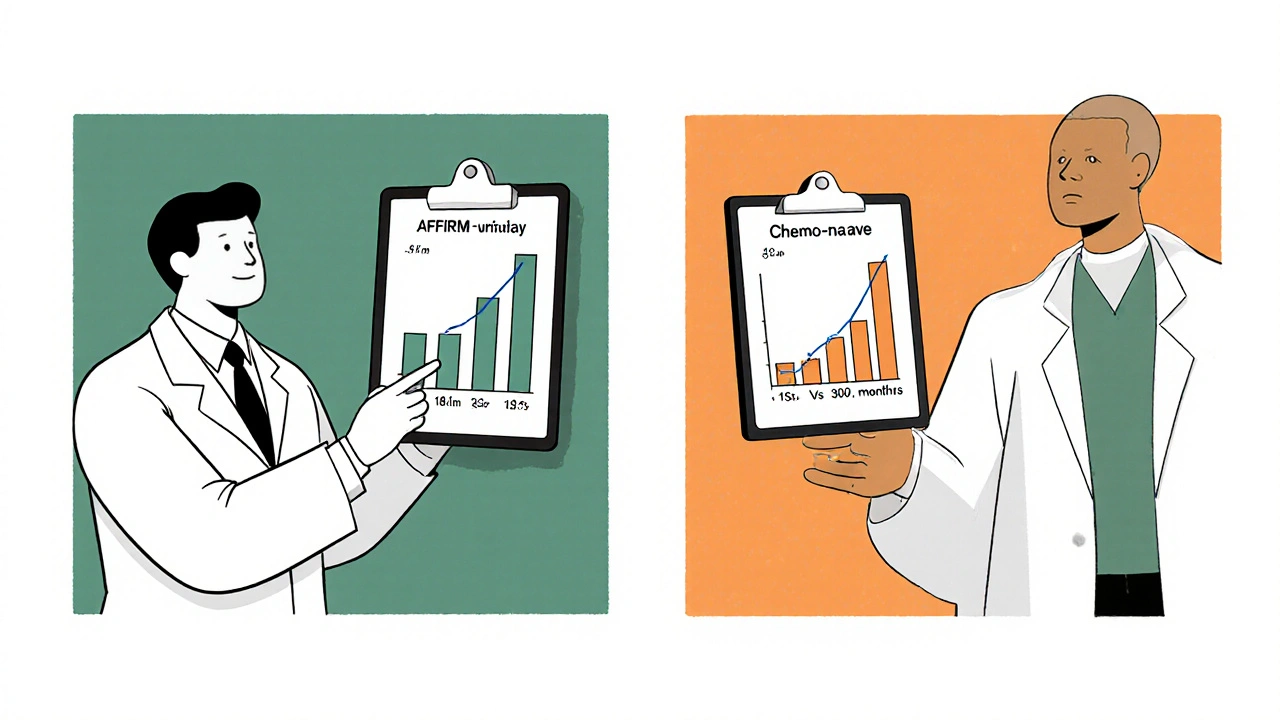
- AFFIRM enrolled men who had already received chemotherapy (docetaxel). Enzalutamide extended median overall survival (OS) from 13.6 months (placebo) to 18.4 months, a 31% reduction in the risk of death (hazard ratio = 0.69).
- PREVAIL focused on chemotherapy‑naïve patients. The trial showed a median OS of 32.4 months with Enzalutamide versus 30.2 months with placebo, translating to a 28% lower death risk (hazard ratio = 0.71).
Both studies required continuous ADT, confirming that Enzalutamide works best when paired with hormone suppression.
| Trial | Population | Median OS (months) | Hazard Ratio (death) | Key Inclusion |
|---|---|---|---|---|
| AFFIRM | Post‑docetaxel mCRPC | Enzalutamide: 18.4 vs Placebo: 13.6 | 0.69 | Prior chemotherapy, ongoing ADT |
| PREVAIL | Chemo‑naïve mCRPC | Enzalutamide: 32.4 vs Placebo: 30.2 | 0.71 | No prior chemotherapy, ongoing ADT |
Impact on Overall Survival
The term Enzalutamide overall survival often pops up in research papers because it captures the endpoint most patients care about. Across both AFFIRM and PREVAIL, Enzalutamide added roughly 3-5 months to median OS compared with placebo. While a few months might sound modest, the benefit is consistent, and many patients experience prolonged time without disease progression.
Beyond median numbers, the drug also delayed radiographic progression by about 6 months in PREVAIL, meaning fewer scans showing tumor growth. In practice, this translates into more quality time-less pain, fewer hospital visits, and a chance to stay active.
Who Benefits Most?
Enzalutamide isn’t a one‑size‑fits‑all pill. Several factors influence who sees the biggest OS boost:
- Performance status: Patients with an ECOG score of 0‑1 (fully active or restricted in physically strenuous activity) tend to gain more.
- AR‑V7 biomarker: The presence of AR‑V7 splice variants in circulating tumor cells predicts poorer response to Enzalutamide. Testing for AR‑V7 can guide clinicians toward chemotherapy if the variant is present.
- Baseline PSA levels: Lower PSA (<10 ng/mL) at treatment start correlates with longer OS.
- Prior treatment history: Chemotherapy‑naïve patients (as in PREVAIL) generally see a bigger absolute OS increase.
In short, a fit patient with low AR‑V7 expression, moderate PSA, and no prior chemotherapy is the ideal candidate.
Safety Profile and Side Effects
Every drug balances benefits with risks. Enzalutamide’s safety data from the large trials show:
- Fatigue (≈30% of patients) - usually mild to moderate.
- Hypertension (≈12%) - monitor blood pressure regularly.
- Seizures (≈0.6%) - rare, but higher risk in patients with a history of seizures or brain metastases.
- Falls and fractures - linked to fatigue and muscle weakness; encourage strength training.
Most adverse events are manageable with dose adjustments or supportive care. Importantly, the drug does not significantly increase liver‑enzyme toxicity, making it easier to combine with other systemic therapies.
Practical Considerations for Patients and Clinicians
Putting Enzalutamide into real‑world practice involves a few logistical steps:
- Confirm continuous ADT (LHRH agonist or antagonist) is in place.
- Start Enzalutamide at 160 mg orally once daily, with or without food.
- Schedule baseline labs: CBC, CMP, PSA, and blood pressure.
- Re‑check labs every 4‑6 weeks for the first two months, then every 3‑4 months.
- Assess for seizure risk - avoid concomitant drugs that lower seizure threshold.
- Consider bone‑protective agents (e.g., zoledronic acid) if the patient has bone metastases.
Cost can be a barrier; many health systems provide subsidies for advanced prostate cancer drugs, and patient assistance programs are available through the manufacturer.
Bottom Line
If you or a loved one is fighting metastatic prostate cancer, Enzalutamide offers a proven way to add months-sometimes years-of life while keeping quality in check. The biggest survival gains appear in patients who are still fit, haven’t yet undergone chemotherapy, and lack the AR‑V7 splice variant. Discuss biomarker testing, side‑effect monitoring, and insurance coverage with your oncologist to make an informed decision.
What is the typical survival benefit of Enzalutamide?
Across the AFFIRM and PREVAIL trials, Enzalutamide added about 3‑5 months to median overall survival compared with placebo, and reduced the risk of death by roughly 30%.
Can Enzalutamide be used before chemotherapy?
Yes. The PREVAIL trial enrolled chemotherapy‑naïve men and showed a clear OS advantage, establishing Enzalutamide as a first‑line option after ADT.
What are the most common side effects?
Fatigue, high blood pressure, and occasional seizures are the main concerns. Fatigue affects about a third of patients, hypertension occurs in roughly 12%, and seizures are rare (≤1%).
Should I get tested for the AR‑V7 biomarker?
Testing for AR‑V7 can help decide whether Enzalutamide is likely to work. Presence of AR‑V7 suggests lower response rates, so clinicians may favor chemotherapy instead.
How is Enzalutamide taken?
The standard dose is 160 mg taken orally once daily, usually alongside continuous ADT. It can be taken with or without food, but consistency helps keep blood levels stable.



Ed Mahoney
Oh great, another 'miracle pill' that adds a few months.
Guess we’re all thrilled to hear about another 3‑month OS bump.
Shermaine Davis
I totally get why patients feel hopeful; those extra months can mean more time with family.
It’s nice to see data backing up the optimism.
Nicholai Battistino
The data clearly shows a modest OS improvement in both trials.
Andrew Wilson
We should be cautious about celebrating a drug that brings side effects like hypertension and fatigue.
Especially when it’s marketed as a cure‑all, the ethical line gets blurry.
Patients deserve transparent risk‑benefit discussions.
Kristin Violette
Enzalutamide’s mechanism of action exemplifies a multi‑modal inhibition of the androgen receptor pathway, which is pivotal in the oncogenic circuitry of metastatic castration‑resistant prostate cancer.
This triple‑lock model-ligand binding blockade, nuclear translocation inhibition, and co‑activator recruitment disruption-creates a robust pharmacodynamic profile.
From a pharmacokinetic standpoint, the 160 mg daily dosing achieves steady‑state concentrations sufficient to maintain receptor saturation.
Clinical trial evidence, particularly from AFFIRM and PREVAIL, demonstrates a consistent median overall survival advantage of approximately 3‑5 months across heterogeneous patient cohorts.
Subgroup analyses reveal that patients with low AR‑V7 expression and an ECOG performance status of 0‑1 derive the most pronounced benefit, underscoring the importance of biomarker‑guided therapy selection.
Conversely, the presence of AR‑V7 splice variants predicts resistance, suggesting a strategic pivot towards taxane chemotherapy in such cases.
Safety data indicate that fatigue, hypertension, and rare seizures constitute the primary adverse event spectrum, necessitating vigilant monitoring protocols.
Hypertension management may involve dose adjustment or adjunct antihypertensive therapy, while seizure prophylaxis remains a consideration for high‑risk individuals.
The drug’s integration with continuous androgen‑deprivation therapy (ADT) reinforces the synergistic paradigm of combined hormonal blockade.
Economically, the cost‑effectiveness ratio is contingent upon health system reimbursement models and patient quality‑adjusted life year (QALY) calculations.
From a health equity perspective, access disparities persist, highlighting the need for policy interventions to broaden availability.
In practice, clinicians should incorporate shared decision‑making frameworks, presenting survival benefit data alongside toxicity profiles.
Future research directions include combination regimens with PARP inhibitors and immunotherapeutic agents to potentially amplify response durability.
Overall, enzalutamide represents a cornerstone in the therapeutic armamentarium, offering a mechanistically sound and clinically validated option for prolonging life in a challenging disease setting.
Theo Asase
This is another example of Big Pharma colluding with the government to push expensive meds on unsuspecting Americans!
They’ll hype a few extra weeks of survival while we shoulder astronomic price tags.
Don’t be fooled by glossy press releases; the real agenda is profit, not patients.
Joey Yap
When we look beyond median survival numbers, we see the lived experience of each patient.
Those incremental months can translate into meaningful milestones-birthday celebrations, school graduations, or simple daily joys.
Thus, the value of enzalutamide isn’t purely statistical; it’s deeply personal.
Lisa Franceschi
I appreciate the thorough presentation of the trial outcomes; however, it is imperative to acknowledge the limitations inherent in subgroup analyses.
Without prospective validation, extrapolating these findings to broader populations may be premature.
Diane Larson
For anyone starting enzalutamide, remember to monitor blood pressure regularly and report any seizure‑like activity immediately.
Staying on top of labs and side‑effect check‑ins can make a big difference in maintaining quality of life.
Michael Kusold
Side effects matter more than a few extra months.