When two drugs are combined into one pill or injection, doctors expect the result to work the same way every time-no matter which brand or generic version is used. But what happens when the doses aren’t identical, or the inactive ingredients change? Therapeutic equivalence isn’t just a label on a bottle. It’s a promise that the medicine will do what it’s supposed to do, safely and consistently. For combination products, that promise gets complicated fast.
What Therapeutic Equivalence Really Means
Therapeutic equivalence means two drug products have the same active ingredients, in the same amounts, delivered the same way, and produce the same clinical results. The FDA uses this to decide if a generic version can safely replace a brand-name drug. In the Orange Book, products rated 'A' are considered interchangeable. As of 2023, over 14,000 drugs carry this rating, and 95% of them are generics that save patients and insurers billions each year. But here’s the catch: therapeutic equivalence only applies when everything matches exactly-active ingredient, strength, dosage form, and route. If you switch from a 10mg/5mg combination to a 5mg/2.5mg version and double the pill count, you’re not following therapeutic equivalence rules. You’re guessing.Why Dose Differences Break the Equivalence Rule
Combination products like amlodipine/benazepril for high blood pressure or ezetimibe/simvastatin for cholesterol are designed to work together. But not all combinations are created equal. Some generics use different ratios. A patient might get switched from a 10/20mg version to a 5/10mg version, and be told it’s "the same thing." But if the patient was stabilized on the higher dose, halving both components could reduce effectiveness. In 2022, the FDA’s adverse event database recorded 247 incidents tied to dose conversion errors in combination products. Nearly 40% involved cardiovascular drugs. One nurse practitioner reported a patient’s LDL jumped 15% after switching from brand-name Vytorin to a generic equivalent-even though both were rated 'A.' The difference? Inactive ingredients altered how the drugs were absorbed.NTI Drugs in Combinations: A High-Stakes Game
Some drugs have a narrow therapeutic index (NTI). That means the difference between a helpful dose and a toxic one is tiny. Warfarin, levothyroxine, and phenytoin fall into this category. When they’re part of a combination, even small changes can trigger serious side effects. A 2018 study in the Journal of Clinical Endocrinology & Metabolism found that 12% of patients switching between "therapeutically equivalent" levothyroxine products had abnormal thyroid levels within weeks. The FDA requires stricter bioequivalence standards for NTI drugs-90-111% instead of the usual 80-125%. But that still leaves room for variation. In combination products, where two NTI drugs are paired, the risk multiplies.How Combination Products Are Rated
The FDA doesn’t rate combination products the same way it rates single-drug generics. There are three main paths:- ANDA (Abbreviated New Drug Application): Copies an existing approved combination. Usually gets an 'A' rating if bioequivalence is proven.
- 505(b)(2) NDA: Makes changes to an existing product-like a new ratio or different inactive ingredient. May get an 'A' or 'B' rating. A 'B' means the FDA isn’t sure it’s interchangeable.
- Petitioned ANDA: A manufacturer asks for an 'A' rating despite differences. Rare, and often rejected unless strong data supports equivalence.
Real-World Mistakes Happen Because of Assumptions
Pharmacists aren’t the problem. The system is. A pharmacist on Reddit shared that in six months, they made three dosing errors with amlodipine/benazepril combinations because different manufacturers labeled the same strength differently. One company called it "10/20," another called it "10mg/20mg"-but the pill looked identical. The pharmacist assumed they were the same. They weren’t. In hospitals, automated systems sometimes swap combination products based on cost, not clinical stability. A patient on a stable dose of tramadol/acetaminophen for chronic pain might get switched to a different generic. The total dose is the same-but the ratio of tramadol to acetaminophen changed. Tramadol’s effect isn’t linear. A small change in its concentration can make pain control worse-or trigger seizures.What Works: Systems That Prevent Errors
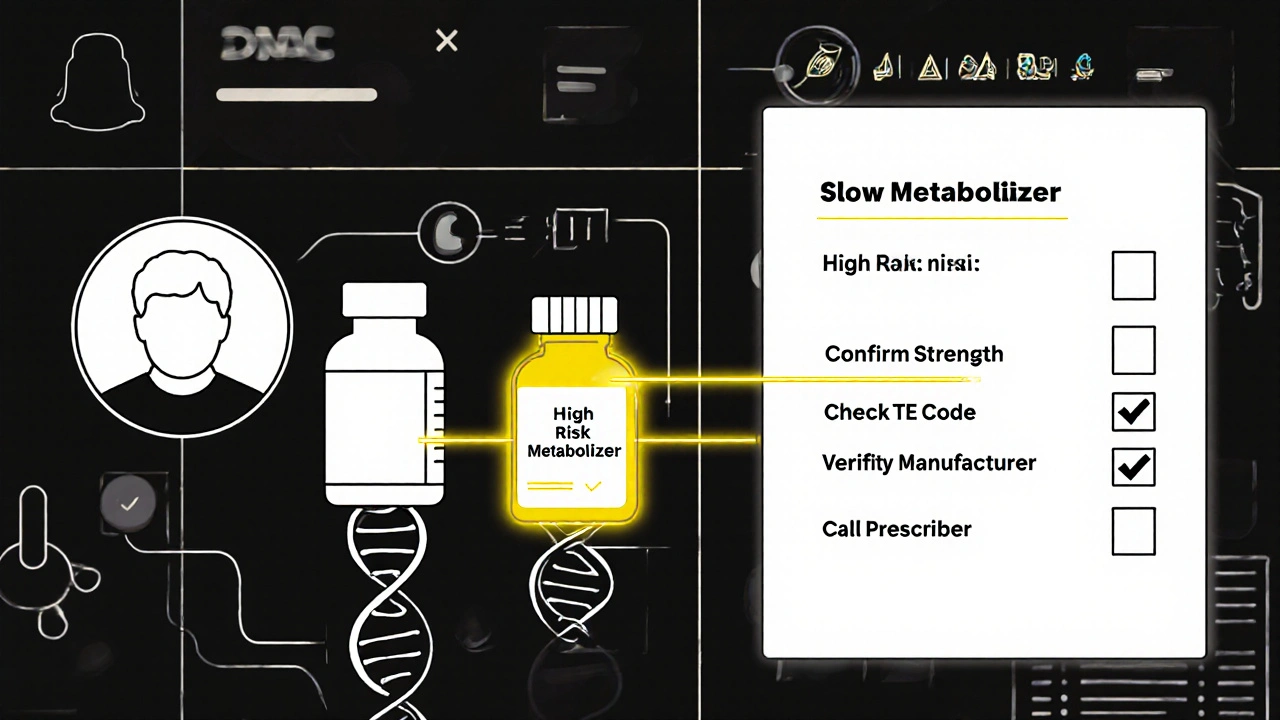
The University of California Health System trained staff for 40 hours on therapeutic equivalence in combinations. They created a checklist:- Confirm the exact active ingredients and strengths match the original prescription.
- Check the Orange Book TE code-not just the name.
- Verify the manufacturer and inactive ingredients if the patient has had prior reactions.
- For NTI combinations, require physician approval before substitution.
- Use barcode scanning on every dispensed combination product.
The Future: Personalized Equivalence?
The FDA is testing machine learning tools to predict which combination products might fail in real patients. Early results show 89% accuracy in flagging substitutions likely to cause problems. By 2030, the NIH predicts that 30% of therapeutic equivalence decisions will include pharmacogenomic data. That means your genes will help determine whether a "generic equivalent" is safe for you. Someone who metabolizes drugs slowly might need a lower dose-even if the pill looks identical to someone else’s. For now, though, we’re stuck with a system built on averages. It works for most people. But not all.What You Should Do
If you’re a patient:- Ask your pharmacist: "Is this the exact same strength and ratio as before?"
- Check the pill’s imprint code and color. Even small changes can mean a different manufacturer.
- Report any new side effects after a switch-even if it’s "the same drug."
- Write the exact brand or generic name, strength, and ratio. Don’t just write "amlodipine/benazepril."
- For NTI combinations, avoid substitutions unless absolutely necessary.
- Document why you chose a specific version. If a patient does poorly after a switch, you’ll need that record.
- Never assume two "A-rated" products are interchangeable if they’re from different manufacturers.
- Use the Orange Book’s TE code-not just the product name.
- When in doubt, call the prescriber. It’s better to delay a fill than risk harm.
Bottom Line
Therapeutic equivalence is a powerful tool to cut costs and improve access. But in combination products, it’s not a free pass to swap anything labeled "generic." Dose ratios, inactive ingredients, and individual biology matter. The system works well for most-but it fails when we treat it like a one-size-fits-all rule. The goal isn’t just to save money. It’s to keep people safe while doing it.Can I swap a generic combination product for the brand name without asking my doctor?
It depends. If the product has an 'A' rating in the FDA’s Orange Book and you’re not taking an NTI drug (like warfarin or levothyroxine), it’s usually safe. But if the combination has multiple active ingredients, or if you’ve had side effects before, always check with your doctor or pharmacist first. Some generics may have different inactive ingredients that affect absorption.
Why do two 'A-rated' combination products sometimes work differently?
Even if two products have the same active ingredients and strengths, their inactive ingredients (like fillers or coatings) can affect how quickly the drug is absorbed. For example, one version might use croscarmellose sodium as a disintegrant, while another uses sodium starch glycolate. In most people, this doesn’t matter. But in patients with sensitive digestive systems or those taking NTI drugs, it can change blood levels enough to cause side effects or reduced effectiveness.
What does an 'A' rating really mean for combination products?
An 'A' rating means the FDA has determined the product is bioequivalent to the brand-name version and meets all safety and strength requirements. But it doesn’t guarantee identical performance in every patient. For combination products, the FDA evaluates each active ingredient separately. If one component has a narrow therapeutic index, the overall product might still be rated 'A' even if the interaction between the two drugs isn’t perfectly predictable.
Are there combination products that can’t be substituted at all?
Yes. Products with a 'B' rating in the Orange Book are not considered interchangeable. These often include 505(b)(2) applications where changes were made to the formulation, route, or strength. Also, some combination products-especially those with biologics or complex release mechanisms-don’t have established therapeutic equivalence ratings at all. Always check the TE code before substituting.
How can I tell if my combination medication has been switched?
Look at the pill’s imprint, color, and shape. If they’ve changed, the manufacturer or formulation likely changed too. Check the label for the manufacturer’s name and the exact strength (e.g., 10mg/20mg, not just "amlodipine/benazepril"). If you’re unsure, ask your pharmacist to confirm the product matches your original prescription. Don’t assume it’s the same just because the name is identical.



Ady Young
I’ve seen this happen in my clinic-patient gets switched from brand to generic combo pill, says they feel ‘off,’ but we assume it’s placebo. Turns out, the new version had a different coating that slowed absorption. Took three weeks to catch it. Now I always check the TE code and manufacturer. Small stuff matters.
Especially with NTI drugs. One wrong pill and someone’s in the ER. It’s not just about cost anymore.
Pharmacists are doing their best, but the system’s broken. We need better labeling, not just more generics.
Travis Freeman
Love this breakdown! Seriously, kudos to the author for calling out how we treat these meds like interchangeable Legos. They’re not! Every time I see someone say ‘it’s just a generic,’ I want to hand them a copy of the Orange Book and a cup of coffee.
Also, the UC Health checklist? That’s gold. We should make that mandatory for every pharmacy chain. Save lives, save money. Win-win.
Sean Slevin
Okay… so… let me get this straight… the FDA says ‘A’ means interchangeable… but then… like… they don’t actually guarantee it? And we’re just… trusting… the system… based on… statistical averages? And… people… are dying? Or… having seizures? Or… their thyroid goes haywire? And… we’re still… okay with this? Like… this isn’t a bug… it’s a feature… of capitalism? We’re optimizing for profit… not for people? And… the machine learning thing… that’s just… a band-aid… on a hemorrhage… right? I mean… if your genes matter… why aren’t we sequencing everyone… before prescribing? Why… are we… still… treating humans… like… data points… in a spreadsheet?!!??
Chris Taylor
My grandma got switched to a generic blood pressure combo last year. She started feeling dizzy and confused. We thought it was aging. Turns out, the new version had a different filler that messed with her stomach absorption. Took her doctor three visits to figure it out. She’s back on the original now. Don’t assume ‘same name’ means ‘same effect.’ Always check.
And yeah, pharmacists are overworked. But they shouldn’t have to be the ones catching these mistakes.
Melissa Michaels
Therapeutic equivalence in combination products remains a critical but underregulated area of pharmacotherapy. The FDA’s current framework relies heavily on bioequivalence metrics that do not account for interpatient variability in pharmacokinetics, particularly in polypharmacy scenarios. The 12% rate of thyroid dysfunction following levothyroxine substitution is not an outlier-it is a predictable outcome of insufficient clinical validation.
Prescribers must specify exact formulations. Pharmacists must verify TE codes. Patients must be educated. No single stakeholder can bear this burden alone.
Standardization is not optional. It is a patient safety imperative.
Nathan Brown
It’s wild how we treat medicine like a commodity. You wouldn’t swap out the engine in your car and say ‘it’s the same brand’ if it didn’t run right. But we do that with pills every day.
And the worst part? The people who get hurt are the ones who can’t afford to fight back. They don’t have time to call their doctor. They don’t know the difference between ‘A’ and ‘B’ in the Orange Book.
Machine learning might help… but it’s still just a tool. The real fix? Stop letting corporations decide what’s safe.
…and yeah, I’m not okay with this system.
😭
Matthew Stanford
Good post. Real talk: if your combo med changes color or shape, don’t swallow it without asking. Simple as that.
And if you’re a doc-write the full name. Don’t just scribble ‘amlodipine benazepril.’ Write 10mg/20mg. Save someone’s life.
Also, barcode scanners? Use them. It’s not hard.
Olivia Currie
OMG I just had a panic attack reading this. I’ve been on the same combo for 8 years. Last month my prescription came in a different pill. I didn’t think twice. Now I’m sweating and Googling ‘can generic meds make you feel like you’re dying’ and I’m crying in my kitchen. I need to call my pharmacist RIGHT NOW. Thank you for this. I’m not crazy. The system is.
Curtis Ryan
Wait so you’re telling me that two pills that look the same and have the same name can be totally different inside? That’s insane. I thought generics were just cheaper versions of the same thing. Not… different magic recipes. My head hurts. I’m gonna go check my pill bottle right now. 😅
Rajiv Vyas
Big Pharma and the FDA are in bed together. This whole ‘therapeutic equivalence’ thing is a scam. The real reason they allow different fillers is so they can patent the next version. They want you addicted to their pills. They don’t care if you get sick. They just want your insurance to pay. You think this is about safety? Nah. It’s about profit. Wake up. The system is rigged.
farhiya jama
Ugh I just spent 20 minutes trying to figure out if my new pill is the same as the old one. I’m tired. I just want to feel normal. Can’t someone just fix this? Why is everything so complicated? I hate medicine.
Astro Service
Why are we letting foreigners make our meds? This is why America is weak. We used to make everything here. Now some guy in India is deciding if my blood pressure pill works. This is why our hospitals are full. We need to ban all foreign generics. Make it American only. Or else we’re all gonna die.