Lopinavir/Ritonavir Drug Interaction Checker
Check Medication Interactions
Interaction Results
Enter a medication name to see interaction details with lopinavir/ritonavir.
When doctors prescribe lopinavir/ritonavir, they’re not just giving two drugs-they’re activating a chemical lock that changes how nearly every other medication in a patient’s body behaves. This isn’t simple pharmacology. It’s a high-stakes game of metabolic control, where one tiny molecule, ritonavir, holds the keys to the entire system. And if you don’t understand how it works, you risk overdose, treatment failure, or even death.
Why Ritonavir Isn’t Just a Partner Drug
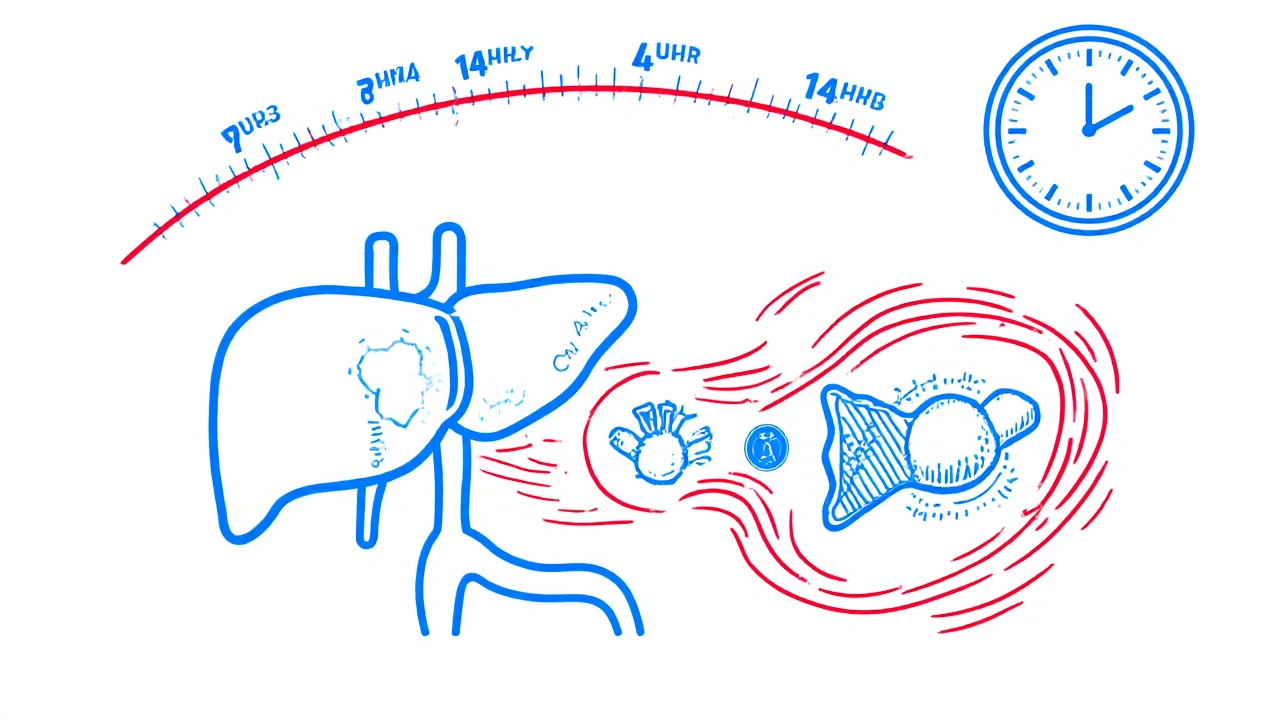
Lopinavir is an antiviral built to fight HIV. But left alone, it gets broken down too fast by the liver. Its half-life? Just under 7 hours. That means a patient would need to take it three times a day to keep levels high enough to suppress the virus. Poor adherence. More side effects. Higher risk of resistance. Enter ritonavir. At 100 mg-just one-fourth the dose needed to treat HIV-it doesn’t fight the virus at all. Instead, it shuts down CYP3A4, the main enzyme in the liver responsible for chewing up lopinavir. This is called pharmacokinetic boosting. Ritonavir doesn’t just slow down metabolism-it permanently disables CYP3A4 molecules by binding to their active site, destroying their heme group, and even sticking its own broken pieces onto the enzyme. The result? Lopinavir’s clearance drops by more than 85%. Its half-life stretches to over 14 hours. Dosing drops to twice daily. Adherence improves. Viral suppression holds. But here’s the catch: ritonavir doesn’t just target CYP3A4. It’s a metabolic wildcard.The Double-Edged Sword of Ritonavir’s Action
Most boosting agents, like cobicistat, are clean. They block CYP3A4 and leave other enzymes alone. Ritonavir? It’s messy. It’s a strong inhibitor of CYP3A4 and CYP2D6-but it’s also an inducer of CYP1A2, CYP2B6, CYP2C9, CYP2C19, and even CYP3A4 itself after prolonged use. This means one drug can do two opposite things at once:- It makes midazolam, fentanyl, and statins stick around 3 to 5 times longer-dangerously so.
- It makes warfarin, oral contraceptives, and voriconazole disappear faster-leaving patients unprotected.
The Interaction Database That Saves Lives
There are over 1,200 documented drug interactions with lopinavir/ritonavir. That’s more than double what you see with newer regimens like darunavir/cobicistat. The Liverpool HIV Interactions Database, updated in July 2023, is the go-to tool for clinicians. It’s accessed nearly 3 million times a year-not because it’s fancy, but because it’s the only thing standing between a patient and disaster. Here’s what it flags as high-risk:- Tacrolimus: Immunosuppressant after transplant. Ritonavir increases levels 4-6x. Dose must be cut by 75%.
- Rivaroxaban: Blood thinner. Contraindicated. Risk of fatal bleeding.
- Methadone: Opioid maintenance. Ritonavir speeds its metabolism. Patients go into withdrawal unless dose is increased by 20-33%.
- Hormonal contraceptives: Effectiveness drops by over 50%. Backup contraception isn’t optional-it’s mandatory.
- Voriconazole: Antifungal. Ritonavir induces its metabolism, but also inhibits it. Levels become unpredictable. Contraindicated.

Why This Still Matters in 2025
In the U.S., lopinavir/ritonavir is nearly extinct. Since 2015, guidelines have pushed doctors toward integrase inhibitors like dolutegravir. They’re simpler, safer, and cause fewer interactions. Less than 5% of new HIV prescriptions in the U.S. now use LPV/r. But globally? It’s still everywhere. In low- and middle-income countries, it makes up 28% of first-line regimens. Why? Cost. A full year of treatment costs $68 through PEPFAR programs. Dolutegravir? $287. When you’re treating millions, price matters more than elegance. And it’s not just HIV. Ritonavir’s boosting power is now the backbone of Paxlovid, the COVID-19 antiviral. Nirmatrelvir, the active ingredient, gets boosted 15-fold by ritonavir. But here’s the twist: Paxlovid’s “rebound” effect-where symptoms return days after treatment-may be linked to ritonavir’s lingering inhibition. Once ritonavir clears (half-life 3-5 hours), CYP3A4 slowly comes back online. If nirmatrelvir levels drop too fast, the virus rebounds. That’s why some experts now recommend extending Paxlovid to five or even seven days in high-risk patients.Real-World Mistakes and How to Avoid Them
The biggest errors aren’t technical-they’re cognitive.- Assuming “boosting” means only inhibition. Ritonavir induces enzymes too. If a patient is on carbamazepine (CYP3A4 inducer), you might think it’ll reduce lopinavir levels. But if they’re also on rifampin (CYP3A4 inducer), levels can drop by 76%. That’s not just a tweak-it’s treatment failure.
- Forgetting about herbal supplements. St. John’s wort induces CYP3A4. A single dose can tank lopinavir levels. Patients don’t always tell you. You have to ask.
- Not checking for delayed effects. When you stop lopinavir/ritonavir, CYP3A4 doesn’t snap back. It takes days to weeks for enzyme activity to return. A patient switched to a new regimen might need lower doses of statins or sedatives for weeks after stopping.
- Ignoring liver function. In Child-Pugh Class B cirrhosis, dose must drop to once daily. Class C? Don’t use it at all. Hepatotoxicity risk jumps to 33% with inducers like rifampin.
What’s Next? The Future of Boosting
Ritonavir’s reign is ending. Newer boosters like cobicistat are cleaner. New HIV drugs don’t need boosting at all. But ritonavir’s role in Paxlovid keeps it alive. Research is now looking at genetic differences-like CYP3A5 expressers-who break down lopinavir faster. Preliminary data shows these patients have 28% lower drug levels. Personalized dosing based on genetics? That’s the next frontier. For now, the message is clear: if you’re prescribing lopinavir/ritonavir, you’re not just managing HIV. You’re managing a metabolic storm. Every drug, every supplement, every lab test-it all connects back to CYP3A4. Miss one piece, and the whole system unravels.When to Avoid Lopinavir/Ritonavir Altogether
There are clear red flags. Don’t use it if the patient:- Takes any drug with a black box warning for CYP3A4 interactions (e.g., alfuzosin, ergot alkaloids, pimozide)
- Has severe liver impairment (Child-Pugh Class C)
- Is on chronic warfarin without daily INR monitoring
- Is pregnant and relying on hormonal birth control
- Has a history of pancreatitis or cardiac conduction issues (both FDA black box warnings)
Is lopinavir/ritonavir still used for HIV today?
Yes, but mostly in low- and middle-income countries where cost matters more than side effects. In the U.S., it’s rarely used for new patients-less than 5% of prescriptions. Integrase inhibitors like dolutegravir are preferred because they’re simpler, safer, and have far fewer drug interactions.
Why is ritonavir used at such a low dose in lopinavir/ritonavir?
Ritonavir at 100 mg doesn’t fight HIV-it blocks CYP3A4, the liver enzyme that breaks down lopinavir. At this low dose, it’s a powerful metabolic inhibitor without causing the side effects (like nausea, diarrhea, liver stress) you’d get at full therapeutic doses. This allows lopinavir to stay in the bloodstream longer, making twice-daily dosing possible.
Can I take statins with lopinavir/ritonavir?
Only certain statins, and only at reduced doses. Rosuvastatin and pravastatin are safest. Atorvastatin can be used at 10 mg daily max. Simvastatin and lovastatin are contraindicated-they can cause life-threatening muscle damage. Always check the Liverpool HIV Interactions Database before prescribing.
Does lopinavir/ritonavir affect birth control?
Yes. Ritonavir reduces the effectiveness of hormonal birth control by more than 50%. This isn’t a small risk-it’s a major one. Patients must use backup contraception (like condoms or an IUD) while on this regimen and for at least 4 weeks after stopping.
What’s the biggest danger when using lopinavir/ritonavir in surgery?
Anesthetic drugs like fentanyl and midazolam can become dangerously potent. Fentanyl exposure can increase by 300%, and midazolam by 500%. Without dose reductions of 60-80%, patients can stop breathing. Anesthesiologists must be notified in advance and adjust all sedatives and pain meds accordingly.
Why was lopinavir/ritonavir tested for COVID-19 and then abandoned?
Early in the pandemic, researchers tried repurposing it because it’s a protease inhibitor and seemed promising in lab studies. But large trials like the RECOVERY study found no benefit in hospitalized patients-mortality was the same as standard care. Later, a better version, nirmatrelvir/ritonavir (Paxlovid), was developed specifically for SARS-CoV-2 and showed 89% efficacy when given early. LPV/r was never designed for coronaviruses.
How long does ritonavir’s effect last after stopping?
Ritonavir’s inhibition of CYP3A4 lasts days to weeks after stopping because it permanently damages enzyme molecules. The liver needs time to make new ones. During this period, other drugs metabolized by CYP3A4 can remain elevated, increasing overdose risk. Doses of statins, sedatives, or immunosuppressants may need to stay lower for up to 2 weeks after discontinuing ritonavir.




Dana Dolan
Man, I read this and thought about my cousin who’s on LPV/r in Kenya. She’s got HIV and takes it with her daily multivitamin-and no one ever told her St. John’s wort in it could tank her levels. She almost got resistance because of a herbal tea. This post is terrifying but necessary.
Also, I just checked the Liverpool database because I’m a nurse and I swear I see this mistake weekly. People forget supplements aren’t ‘harmless.’
Codie Wagers
Let’s be honest-this isn’t pharmacology. It’s alchemy with a side of hubris. We took a drug designed to kill a virus and turned it into a metabolic god that controls the fate of every other molecule in the body. We didn’t cure HIV-we created a pharmacological dictatorship.
And now we’re surprised when patients die? The real tragedy isn’t the interaction-it’s that we still think we can control nature with a pill. We’re not healers. We’re chemists playing god with enzymes we barely understand.
Paige Lund
So… we’re still using this in 2025 because it’s cheaper? Like, we’re choosing between ‘probably won’t kill you’ and ‘definitely will kill you if you forget your birth control’? Sounds like a bargain.
Also, Paxlovid rebound? Yeah, that’s just ritonavir being a chaotic gremlin. We didn’t fix the problem-we just gave it a new name and a patent.
Michael Salmon
This whole post reads like a glorified drug rep’s sales pitch wrapped in a textbook. You’re acting like ritonavir is some magical key when it’s just a blunt instrument. And don’t get me started on the Liverpool database-2.5 million visits a year? That’s not a tool. That’s a symptom of a broken system.
Meanwhile, the real issue? Pharma companies knew this was a mess for 20 years and kept selling it because it was profitable. Now they’re patting themselves on the back for Paxlovid like they invented the wheel. Wake up. This is corporate pharmacology at its worst.
Joe Durham
I work in a clinic that serves a lot of homeless patients on LPV/r. Most of them are on 8+ meds-some OTC, some from the ER, some from their cousin’s ‘natural remedy’ stash. We spend 20 minutes per visit cross-checking because one missed interaction can land someone in ICU.
It’s exhausting. But we do it. Because these people don’t have the luxury of newer drugs. I wish we lived in a world where cost didn’t dictate who gets safe care. But until then? This post? It’s our Bible.
Nick Lesieur
Wait so you’re telling me I can’t take my CBD gummies with Paxlovid? But they’re ‘natural’! 😏
Also, why does everyone act like ritonavir is the villain? It’s just doing what it’s told. The real problem? Doctors who think ‘boosting’ means ‘magic fix.’ You don’t get to ignore 1200 interactions because your patient ‘seems fine.’
Also, St. John’s wort? I’ve seen 3 people crash their HIV meds because of that. And no, they didn’t mention it. Because they think ‘herbal’ = ‘safe.’
Angela Gutschwager
Just took my last dose of LPV/r last week. Switched to dolutegravir. Felt like I got my liver back.
Also, my birth control worked again. 😌
Andy Feltus
There’s a quiet irony here: we built a system where a single molecule, designed to fight one virus, ends up reshaping the entire biochemical landscape of the human body. And we call it medicine.
But maybe that’s the real lesson-not that ritonavir is dangerous, but that we treat biology like a circuit board. We plug in one wire, think we’re fixing a glitch, and suddenly the whole system shorts out.
The future isn’t better drugs. It’s humility. We don’t control metabolism. We just poke it and hope it doesn’t bite back.